Share
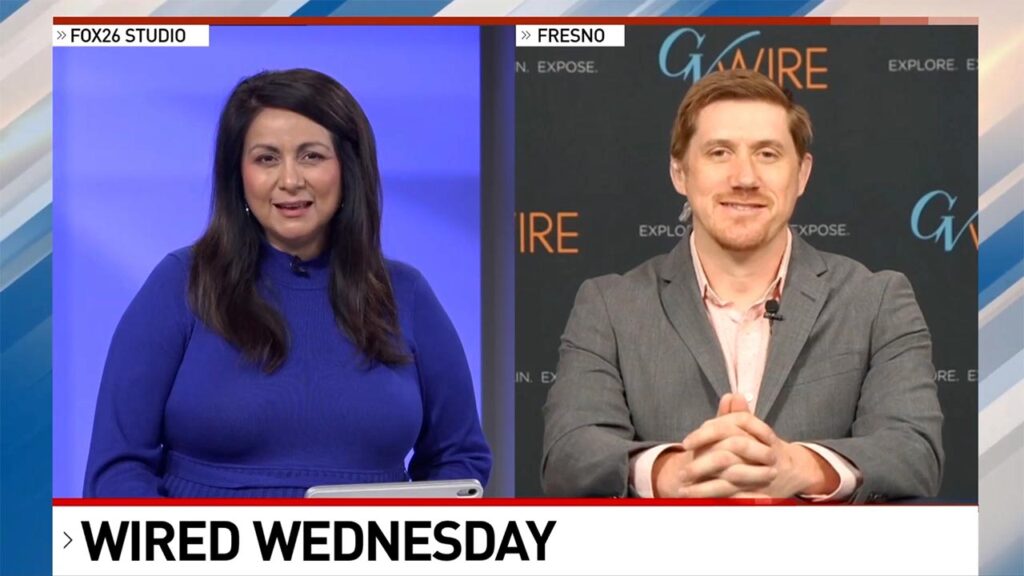
“We almost don’t need them for everybody,” Dr. Roberta Luskin-Hawk says of COVID-19 tests.
Her reasoning is explained below, but it also comes from a wealth of medical experience. She is an infectious disease specialist who worked with Dr. Anthony Fauci in Chicago during the AIDS epidemic. And she is the chief executive for St. Joseph Health in Eureka.
Her hospital just began a clinical trial for the antiviral drug remdesivir and has enrolled one patient so far.
GV Wire spoke to Luskin-Hawk via Zoom on Friday morning.
Q&A with Dr. Luskin-Hawk
GV Wire: Abbott Labs is producing about 50,000 COVID-19 test kits a day. Is it likely we’ll ever get enough test kits for everyone?
A test for someone with no symptoms is pretty meaningless because they could pick it up a week from now. But tests that are also in development are these antibody tests, which will be a blood test that will be simpler and easier to do. It will be for someone who has already been exposed and may have some immunity. So I think there will be combinations of testing, but the need to test for the virus itself is not something we need to do in every person.
Of all the clinical trials you could have joined, why remdesivir?
As an infectious disease doctor, I was intrigued by something that had specific antiviral activity. But I think the other compelling anecdote and it is strictly related to one case which is not sufficient to know how the drug works, but the first case report in the U.S., from Washington state was a man who was in ICU and was given remdesivir. This was reported in the New England Journal of Medicine … and the patient went on to do well.
What does remdesivir do?
These drugs interfere with (COVID-19) multiplying. This particular drug was developed in part to treat ebolavirus and then was studied in two other types of coronaviruses, what’s called SARS and MERS and both of them are kind of causes of the novel coronavirus we’re dealing with now.
How many patients are in the remdesivir trial?
We’ve enrolled one person so far. But enrollment across the country is so active these trials might be fully enrolled, even in a couple weeks, the way it’s going.
How has your patient responded?
I understand the patient is doing well but that’s probably all I can say. Because of privacy.
Do you have insights on some of the other vaccine trials?
I don’t have in-depth knowledge. I know there’s a vaccine that was developed in Israel that I understand may start trial as early as June. … There are many people trying to develop vaccines.
[covid-19-tracker]
Coronavirus Patients Rush to Join Studies of Gilead Drug
Coronavirus patients around the world have been rushing to join remdesivir studies that opened in hospitals in the last few weeks.
Interest has been so great that the U.S. National Institutes of Health is expanding its study, which has nearly reached its initial goal of 440 patients. The drug’s maker, California-based Gilead Sciences, is quickly ramping up its own studies, too.
Remdesivir is given through an IV. It’s designed to interfere with an enzyme that reproduces viral genetic material.
Gilead supplied remdesivir for two studies in China. Results are expected by the end of April.
Cost to Produce the Medicines
According to Reuters, a study released Friday in the Journal of Virus Eradication, researchers, including Howard University chemist Joseph Fortunak, examined the cost of manufacturing medicines in recent or ongoing COVID-19 trials.
Using prices for active pharmaceutical ingredients to build estimates, they said Gilead Science’s experimental drug remdesivir, originally for Ebola, could be made for as little as $0.93 for a day’s supply.
Gilead said the figure does not “accurately reflect” manufacturing costs at scale, but it did not give those costs.
Gilead can produce 140,000 remdesivir treatment courses near-term, and 1 million-plus by December, it has projected.
RELATED TOPICS:
Categories
Canada Presses OpenAI for Answers on Mass Shooter’s Chatbot Use